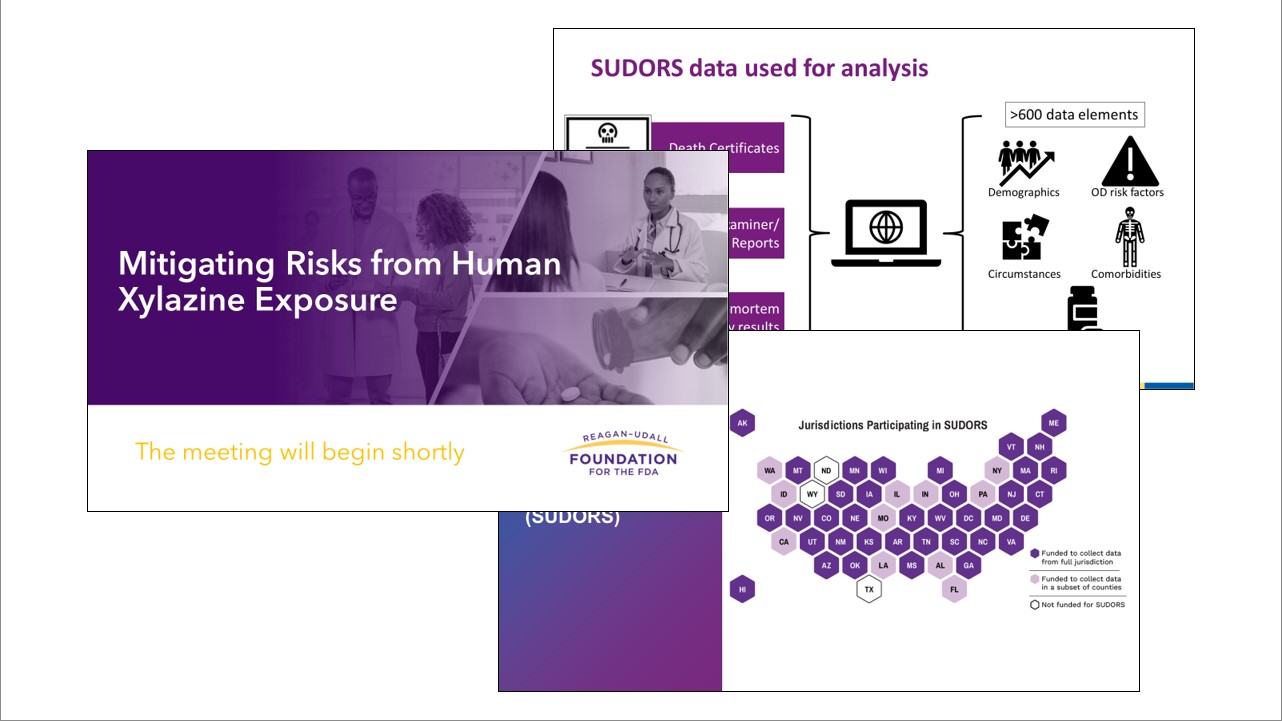
Mitigating Risks from Human Xylazine Exposure
Public Meeting: In-person and Virtual
October 4, 2023 | 9:15 am-5 pm (eastern)
The Foundation partnered with the Food and Drug Administration (FDA) to host "Mitigating Risks from Human Xylazine Exposure," a day-long meeting with both in-person and virtual attendance.
This public meeting explored real-world experiences and scientific evidence on emerging data trends for human xylazine exposure and examined concrete strategies for drug development and clinical research that directly supports the mitigation and reduction of risks associated with human exposure to xylazine. Presenters and discussants included clinical and scientific experts, community and harm reduction organizations, academic researchers, and federal partners. 
Xylazine is a non-opiate sedative, analgesic, and muscle relaxant only authorized in the United States for veterinary use, as approved by the FDA. It is not currently a controlled substance under the U.S. Controlled Substances Act, nor is it approved for human-use. However, human exposure of xylazine has emerged as a growing public health issue. Last year, the Drug Enforcement Administration reported that forensic laboratory identifications of xylazine have risen in all four U.S. Census regions between 2020 and 2021. Furthermore, due to its impact on the opioid crisis, fentanyl mixed (adulterated) with xylazine has also been declared an emerging threat by the White House’s Office of National Drug Control Policy. Given this emerging threat, FDA believes a better understanding of the landscape of available tools and preventive strategies for reducing illicit use of xylazine is needed to advance the development and access to evidence-based treatment for human exposure.
Agenda
| 9:15am | Welcome |
|---|---|
| 9:25am | Opening Remarks: Public Health Strategy |
| 9:40am | Overview of Xylazine and Addressing Data/Research Gaps |
| 9:55am | Current Landscape and Epidemiological Trends |
| 11:05am | Pharmacological and Clinical Research Needs |
| 12:05pm | Lunch Break (on your own) |
| 1:10pm | Opportunities to Address Product Development Research Needs |
| 2:20pm | On the Ground Response to Xylazine |
| 3:35pm | Future Directions |
| 4:45pm | Closing Remarks |
| 5pm | Adjourn |