The FDA encourages the leveraging of Sentinel capabilities through the Innovation in Medical Evidence and Development Surveillance (IMEDS) program as a national resource for broader public health and medical evidence generation. Besides surveillance activities, the IMEDS distributed database can be used for population characterization and effectiveness studies. IMEDS projects must be scientifically suitable for use by the database and should address a safety concern of public health interest. Examples are exposures to medical products, exposure-outcome relationships, or impact of risk mitigation activities.
The Reagan-Udall Foundation provides educational opportunities to learn more about how to use the IMEDS database to answer important post-market research questions. Please contact us directly at IMEDS@reaganudall.org for more information.
If you have an idea for a research project, or are interested in learning how to leverage IMEDS as a resource for evidence generation, fill out this brief form to start the conversation.
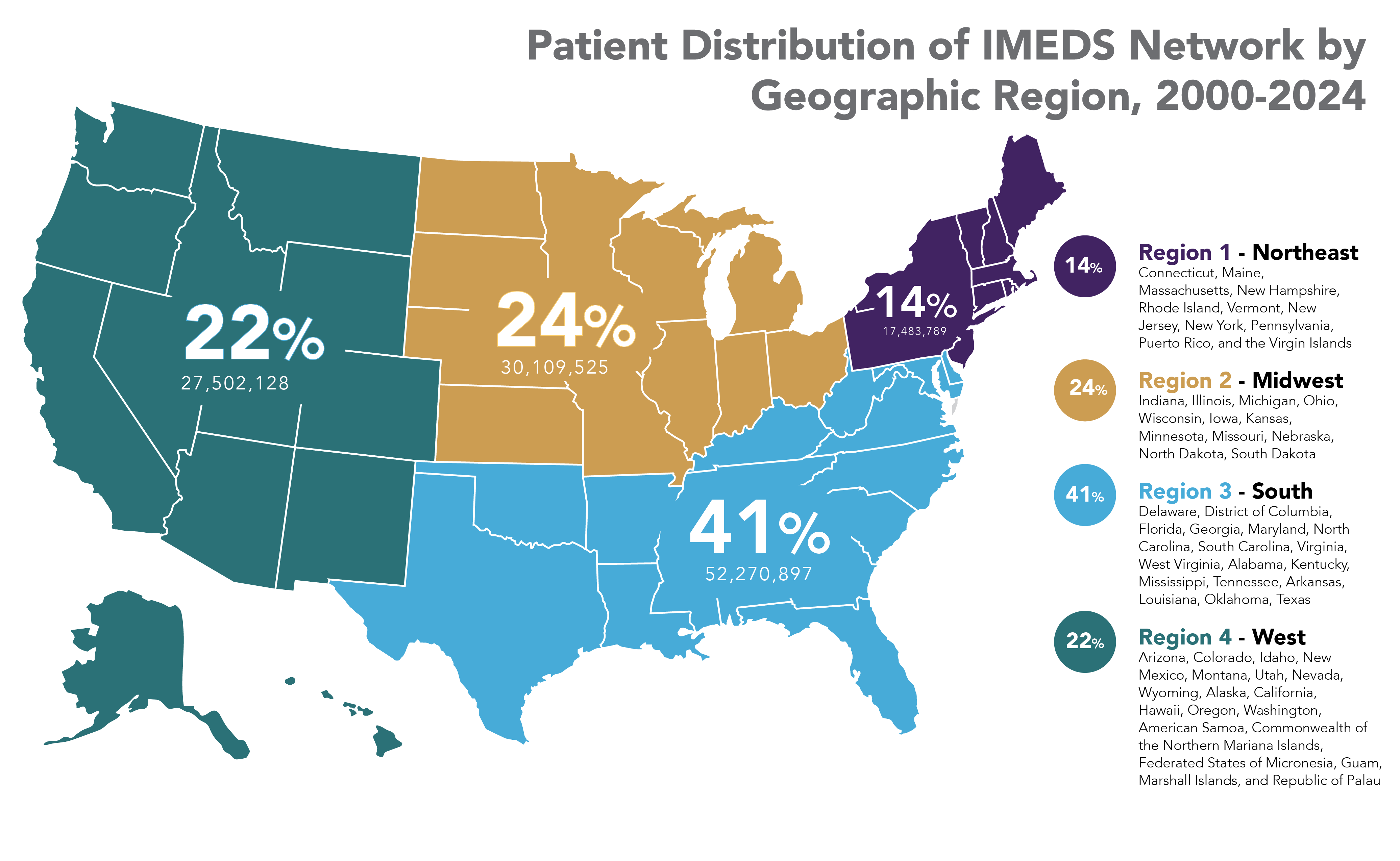
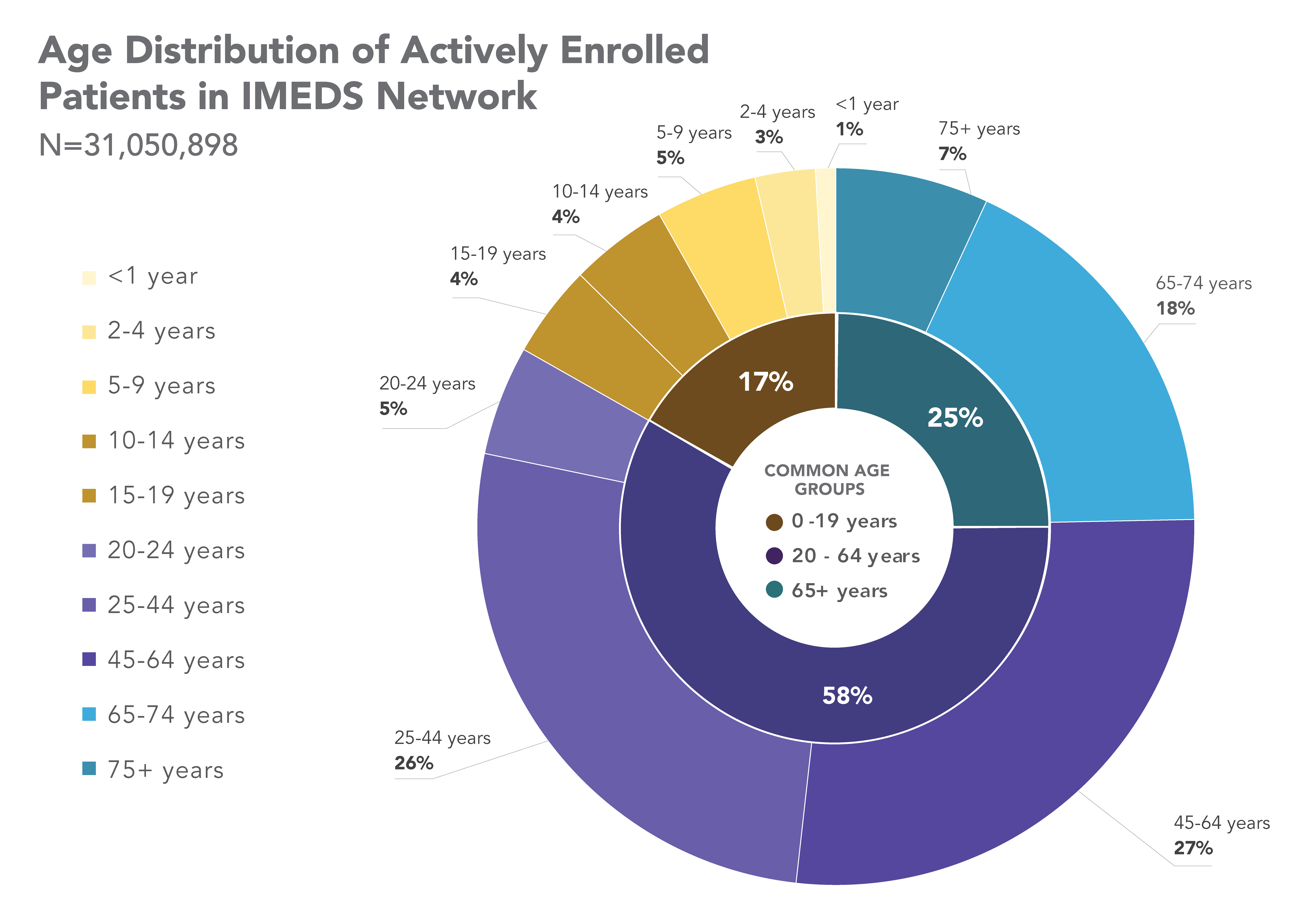
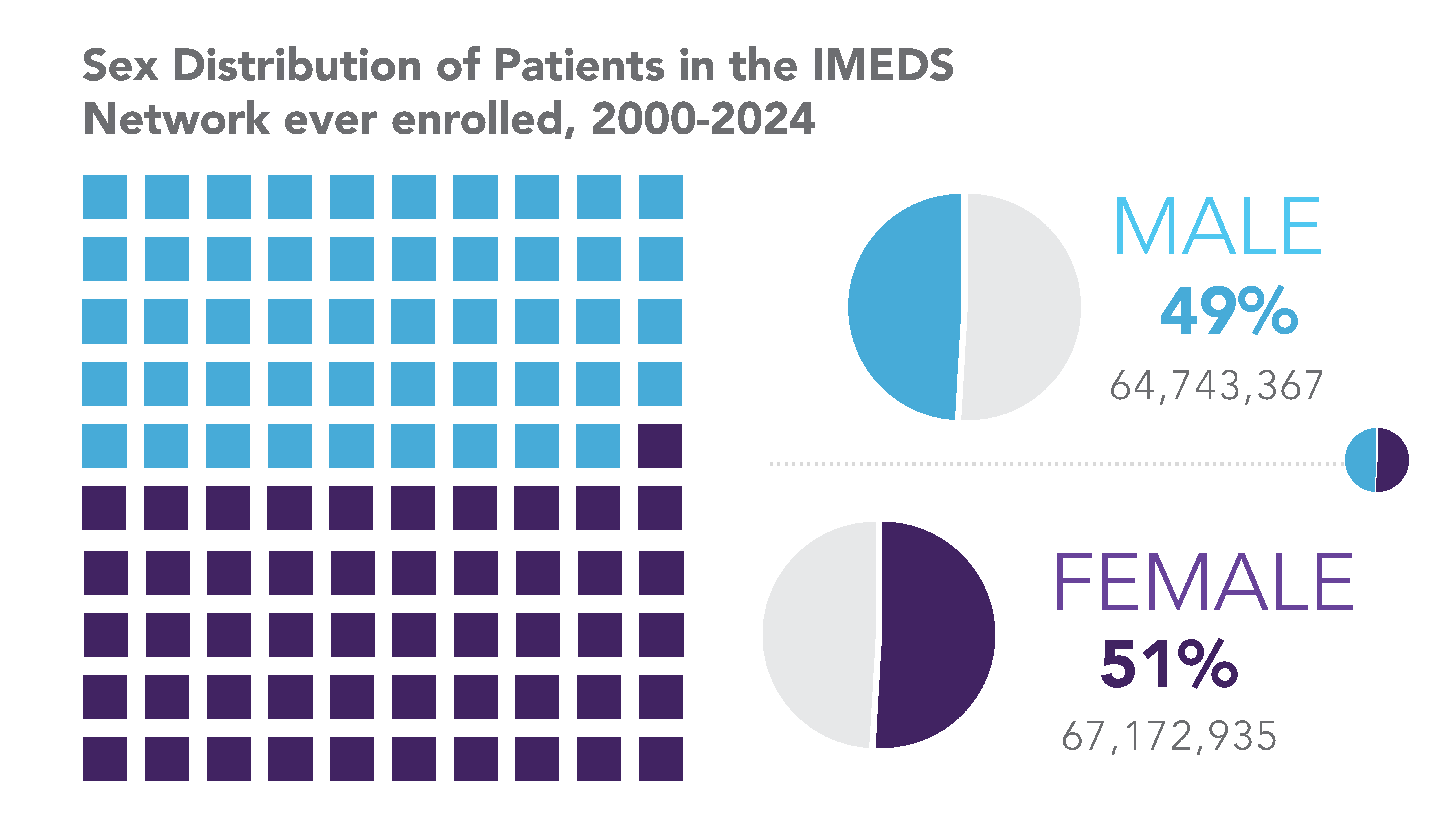
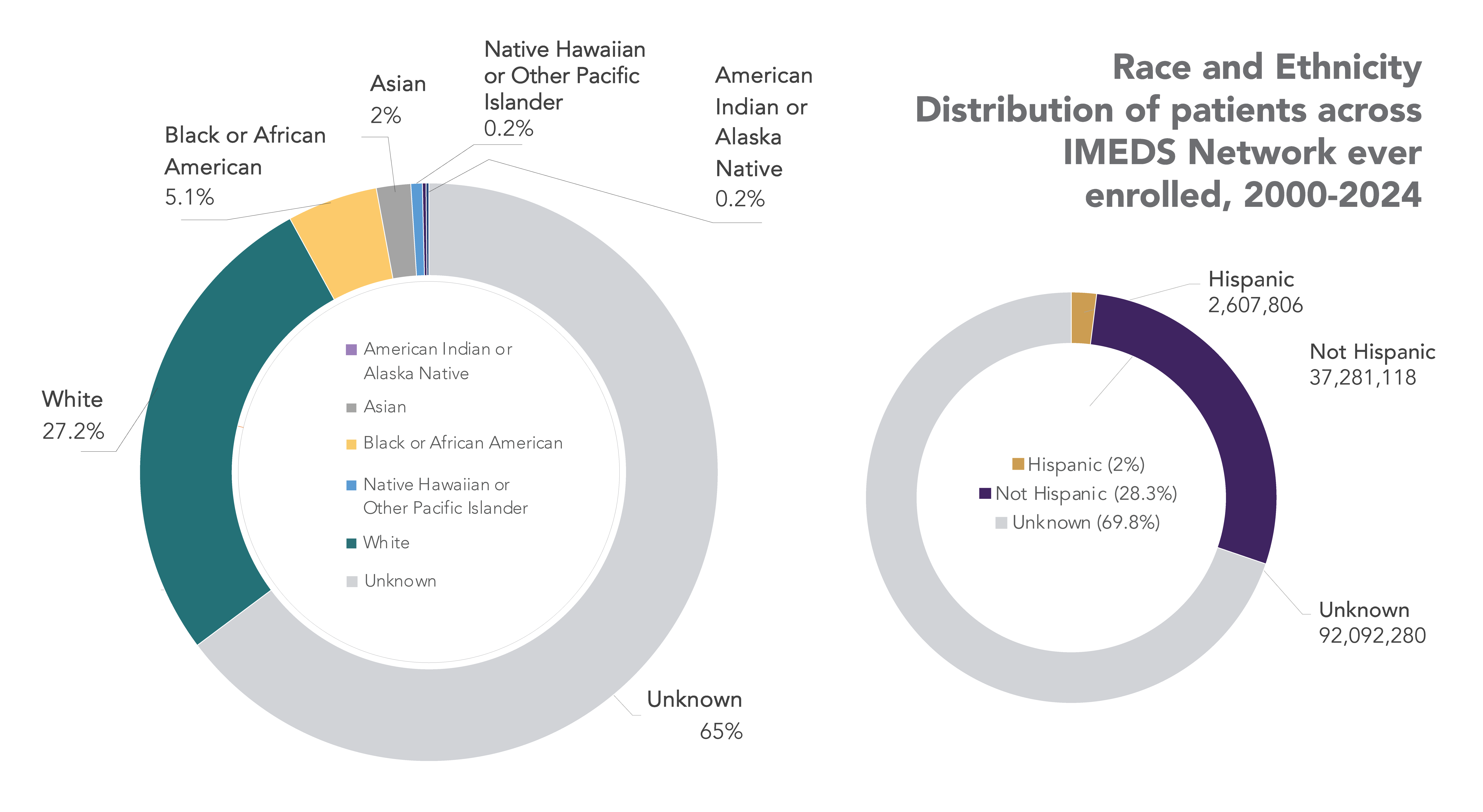
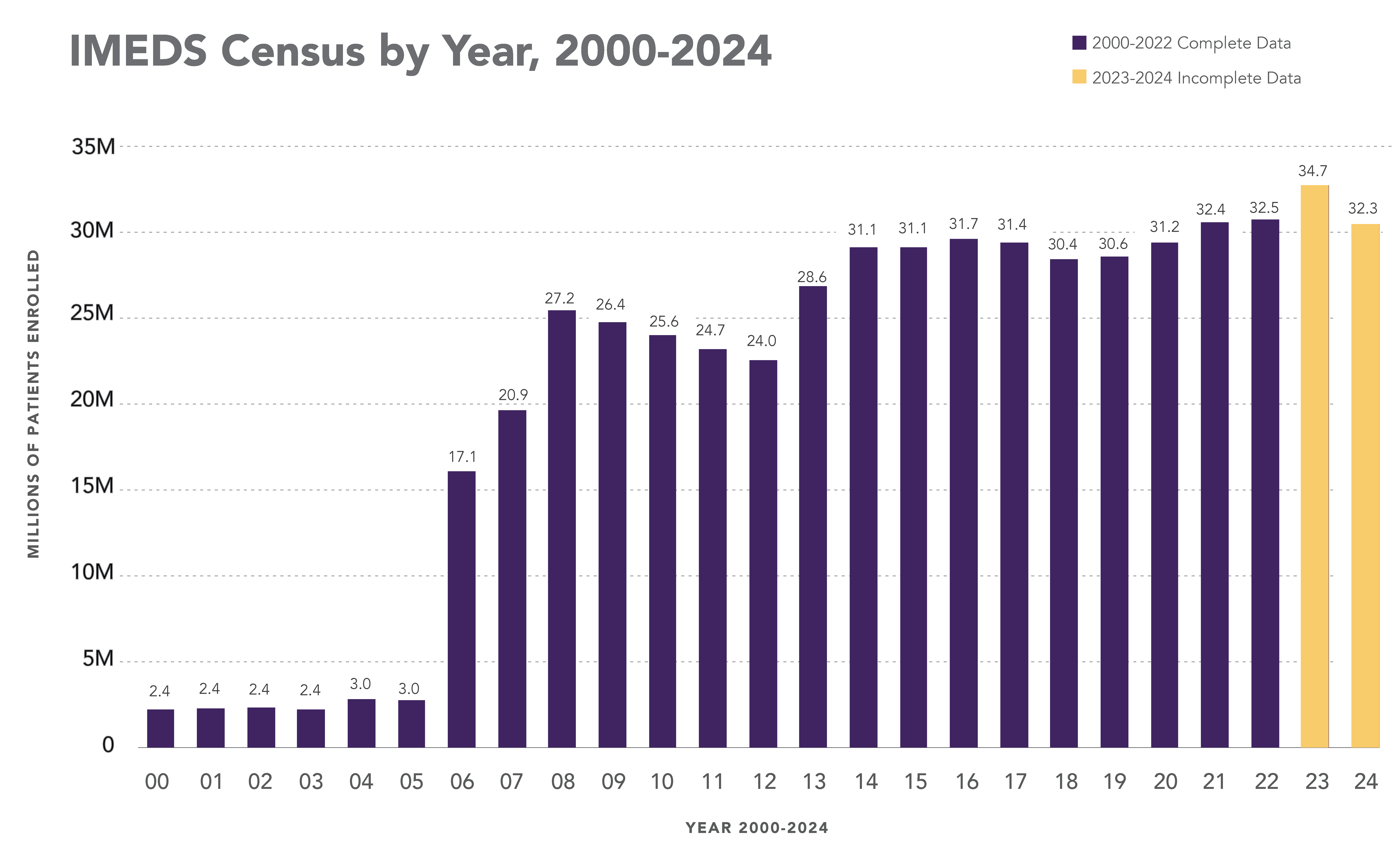
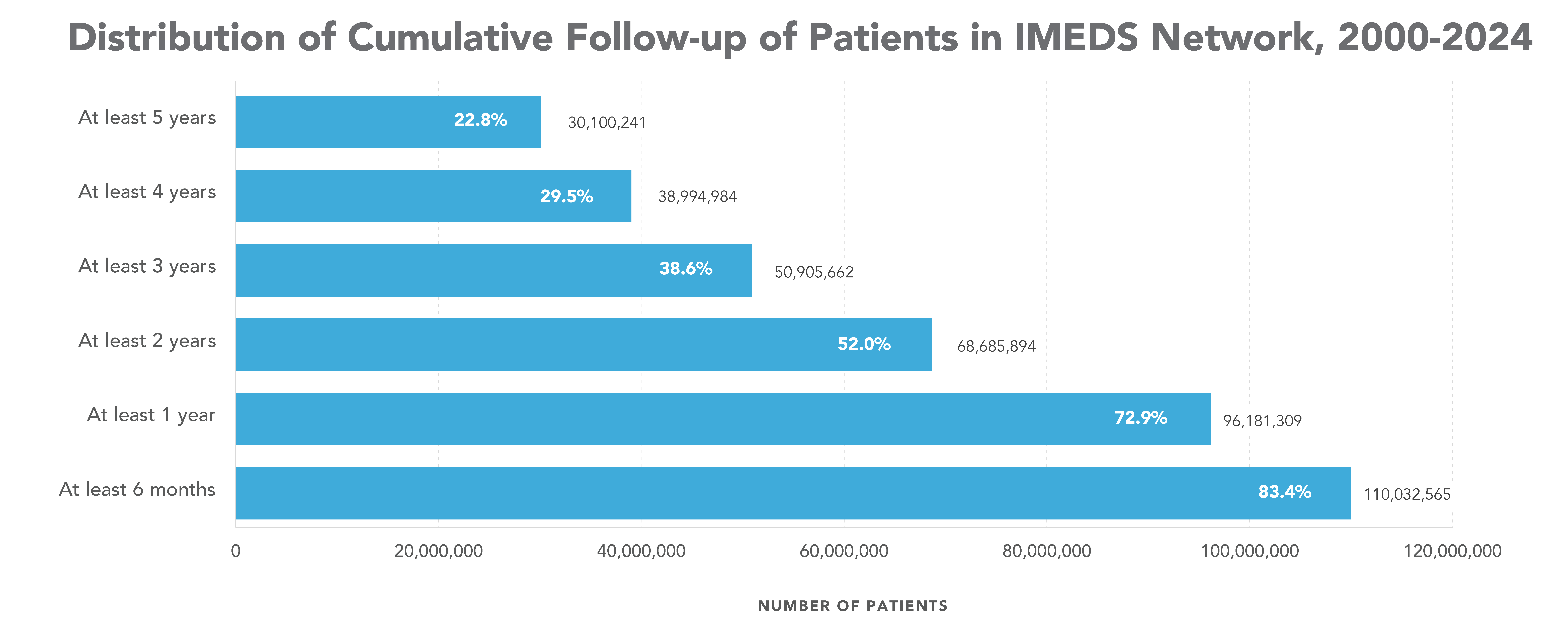
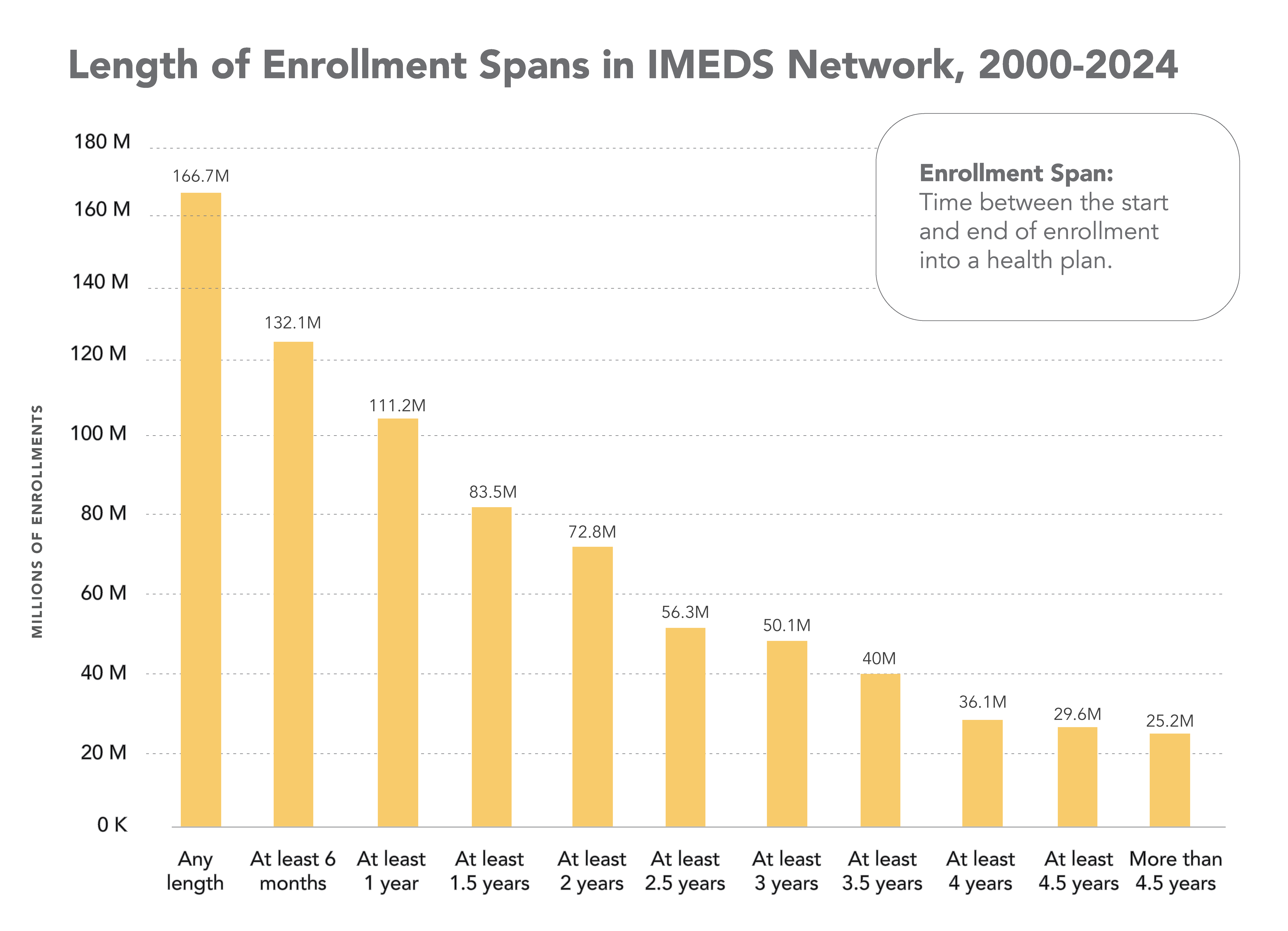
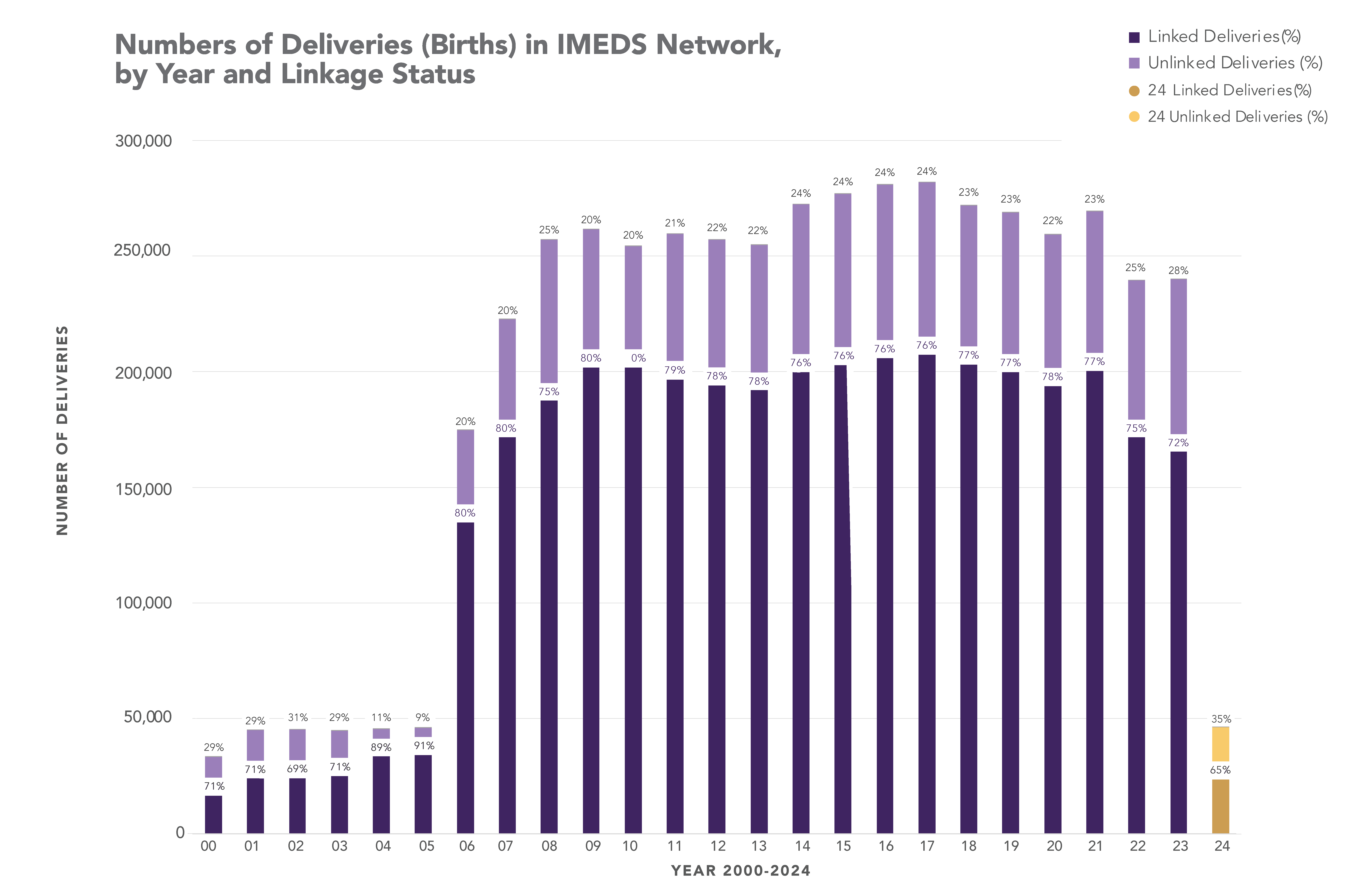
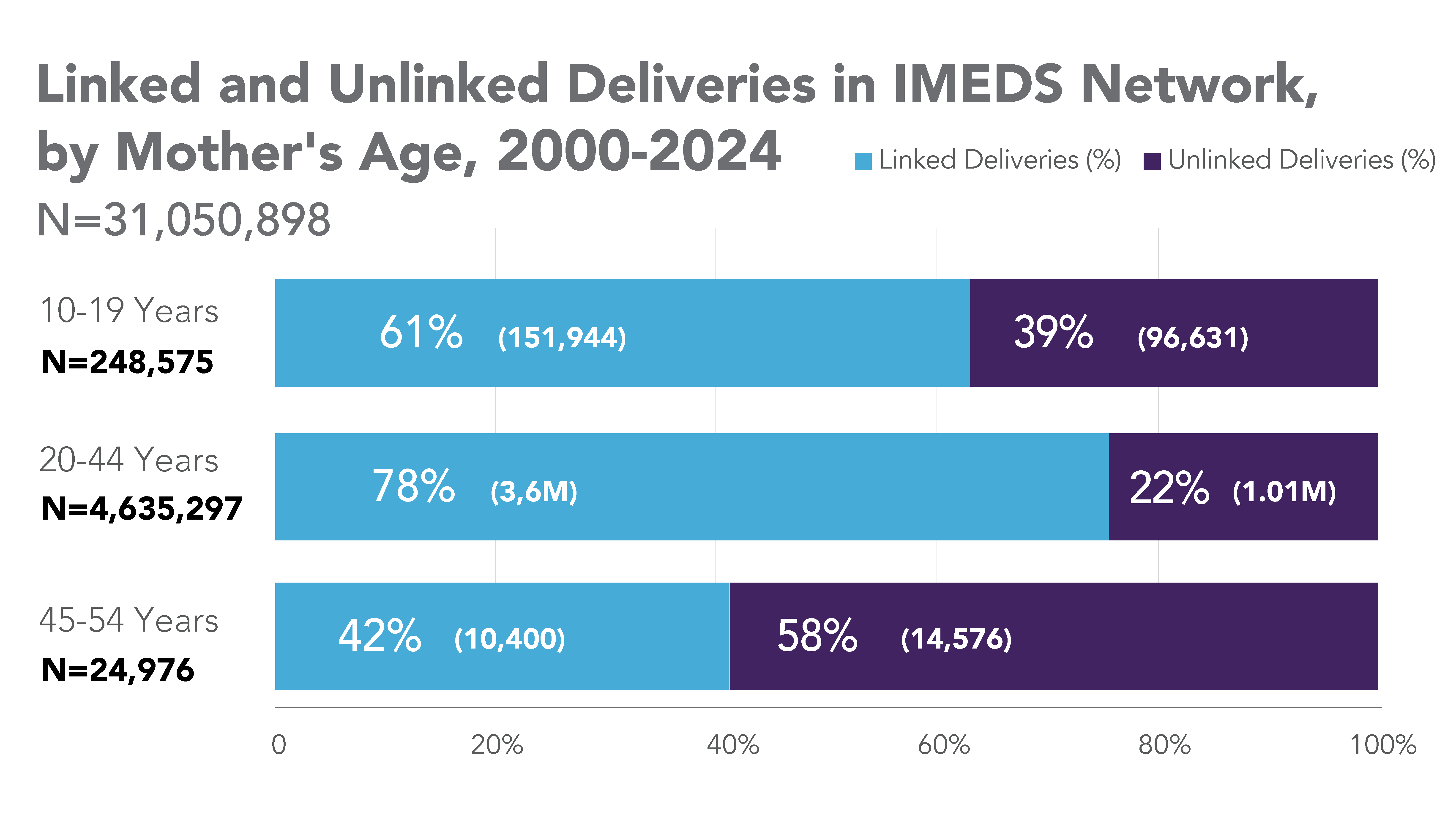
IMEDS Distributed Network includes nine Network Partners (NP), with the majority of the IMEDS NPs overlaps with the Sentinel Distributed Data Network and contribute the data and scientific expertise IMEDS leverages to conduct real-world evidence projects including Post Market Required (PMR) studies. The graphs below represent data from seven network partners from 2000 through their most recently available data as of July 2023. The recency of data varied across NPs, with the most recent data available ranging from December 2021 – July 2023. Queries were run using Sentinel distributed query tools.
For more on IMEDS, watch our Introduction to Innovation in Medical Evidence and Development Surveillance (IMEDS): The Research Resource webinar here.