2020 Annual Public Meeting Highlights
The Foundation convened its annual public meeting of the Board of Directors on October 13, 2020, with nearly 180 stakeholders in virtual attendance. Our message was focused on how we could best leverage our collaborative knowledge to inform the future of public health.
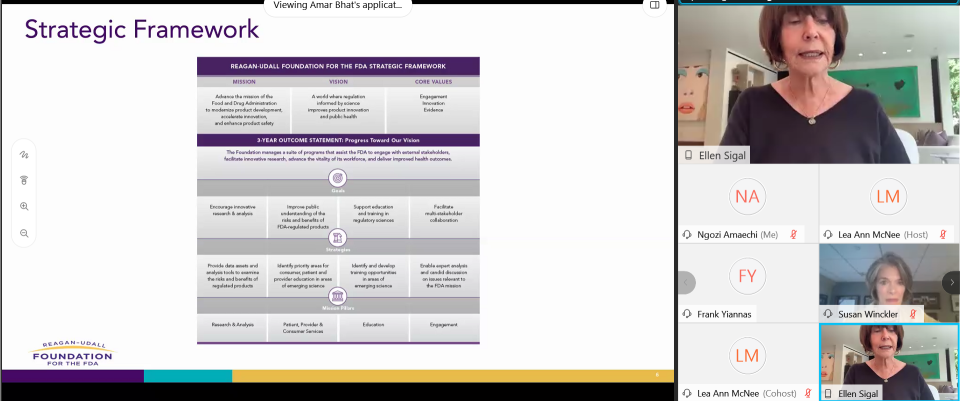
The 2020 Annual Meeting saw the unveiling of the FDA Foundation’s new Strategic Framework and Mission Pillars to help guide the work of the organization. It was also an opportunity to introduce the newly appointed CEO, Susan C. Winckler, RPh, Esq. She joined the organization in May from Leavitt Partners where she served as President.
Winckler moderated a panel discussion between three deputy commissioners for the FDA: Amy Abernethy, MD, PhD, Anand Shah, MD, and Frank Yiannas, MPH. Their conversation centered on what lessons, especially pertaining to public-private partnerships, should be advanced and refined in the ‘new normal’ post-COVID. Dr. Abernethy focused her remarks on the work of the Evidence Accelerator and what that collaborative effort might achieve in the future when focused on health questions other than the coronavirus. Frank Yiannas detailed a blueprint for food safety, and the need for strengthening the partnerships that developed in the past year. Dr. Shah spoke of the innovations spurred by public/private collaboration.
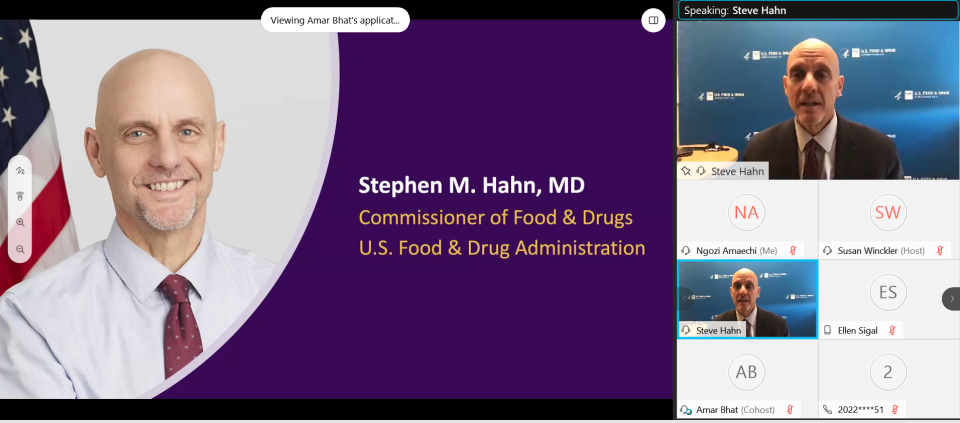
Following the panel, the FDA Foundation Board Chair Dr. Ellen V. Sigal was joined by Commissioner Stephen Hahn who spoke about the FDA’s leadership in the federal response to COVID-19. He referenced the massive effort of the FDA’s medical products centers supporting the development of safe and effective medical countermeasures, from ensuring that front-line health care workers had the necessary protective equipment, to providing essential regulatory advice, guidance, and technical assistance to advance the development of tests, therapies, and vaccines. Hahn also commented on the work FDA did, in conjunction with partners at CDC and OSHA, to help employers and employees dealing with food supply chain issues in the earliest days of the pandemic. He also spoke of the non-COVID priorities of the agency, such as implementing the Food Safety and Modernization Act as well as educational campaigns on nutrition labels and genetically engineered foods.
Hahn applauded the work of the Reagan-Udall Foundation and Friends of Cancer Research on the COVID-19 Evidence Accelerator as “a great example of how a large group of stakeholders can come together to advance the science of real-world data and its use.”